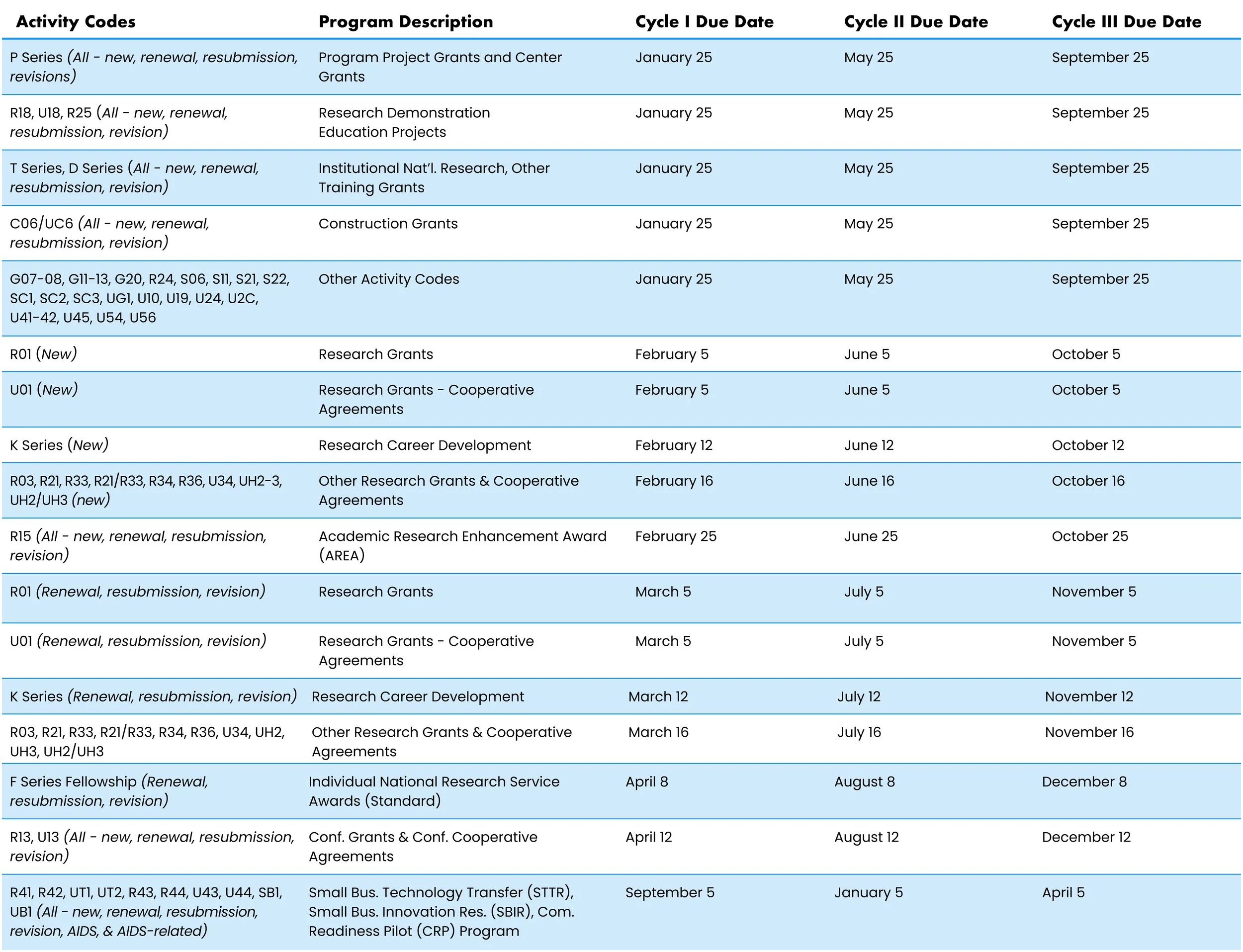
NIH standard due dates
The Key Dates section of many funding opportunities indicate standard dates apply. Use the table below and the activity code (e.g., R01) specified in the title of the opportunity to determine application cycles and their relationship to due dates, review and council dates, and earliest possible start dates. Renewal/resubmission/revision and AIDS-related applications may have different due dates than new applications. Read the table carefully.
General information
Grant applications and associated documents (e.g., reference letters) are due by 5:00 PM local time of applicant organization on the specified due date.
Questions related to a specific opportunity should be directed to the IC contact listed in Section VII of the funding opportunity.
R01, R21 and R34 applications with a PD/PI appointed to an NIH Peer Review advisory group may be eligible for an alternate submission schedule.
See Continuous Submission for details and review assignment cutoff dates.
Application due dates
If you do not see your activity code, check your funding opportunity or the Activity Code Database.
Review and award cycles
Notes:
The actual date of the Advisory Council may occur in the month before or after the month listed. For example, some ICs may actually hold the January Advisory Council meeting in February or the October Advisory Council meeting in September.
Awarding components may not always be able to honor the requested start date of an application. Before incurring any pre-award obligations or expenditures applicants should be aware of NIH policy governing pre-award costs prior to receiving a Notice of Award. See the NIH Grants Policy Statement.
*Advisory Council Round for Cycle I applications (Cycle III for SBIR/STTR) may be August or October, and their earliest project start date may be September or December respectively.